Mammalian cells don’t like to be grown in culture.
Single-celled organisms, like bacteria or yeast, grow beautifully in a nutritious liquid medium, because that’s their natural environment.
Happy yeast cells in a petri dish.
Mammalian cells, by contrast, are meant to grow in, y’know, mammals. They’re expecting to find themselves integrated into complex tissues, full of regulatory chemicals that tell them how to differentiate, when to grow, and when to die. A bioreactor is not their natural home.
Moreover, mammalian cells are not meant to proliferate forever. They get a certain number of cell divisions and then they die. Unless they’re drawn from a tumor, which already proliferates indefinitely, mammalian cells have to be “immortalized” — mutated — to escape cell senescence and become a permanently dividing cell line suitable for cell culture. So mammalian cells in culture are always importantly different from cells as they exist in a living mammal. They’re either cancer cells or artificially cancer-ified cells. And this tends to make them fragile.
The upshot is, mammalian cell culture is a tricky business. Mammalian cells in vitro like to die.
Growing mammalian cells at industrial scale is widely considered to be “more art than science” even by the bioengineers who specialize in it. Cell culture growth is sensitively dependent on the conditions in the bioreactor, and the dependencies are very poorly understood.
How bad is it? Well, for instance, once culture growing conditions have been optimized at one scale (say, a benchtop reactor), they have to start almost from scratch with figuring out how to scale up to a larger size (say, a pilot plant). The effect of something as simple as reactor volume on mammalian cell culture growth is unknown and unpredictable.
Mammalian cell culture is labor-intensive, expensive, and scales very poorly.
This is bad news, because mammalian cell culture is big business, and could (and should) become much bigger.
Applications of Mammalian Cell Culture
What do we use mammalian cells for?
Let’s start with the biggest current market: monoclonal antibody drugs. That’s $150B/year as of 2019. Antibodies are currently the most common kind of “biologic” drug, and biologic drugs are outpacing traditional small molecule drugs because they have higher rates of FDA approval and command higher prices.
A drug company manufactures a monoclonal antibody drug by designing a line of mammalian cells that produces that antibody, growing a whole lot of them, and then extracting the antibody protein from the cells.
For each new antibody drug, a new cell line needs to be devised, and that means starting essentially from scratch in optimizing the cell culture process to maximize cell growth and the abundance of the desired antibody in the culture, and minimize the chance that the whole cell culture dies off.
Manufacturing costs in antibody production are high — hundreds of dollars a gram, hundreds of millions per year for each facility. And manufacturing yields are low — roughly 25% of batches are defective.1 2 A technology which made a serious impact on cell culture efficiency could generate billions of dollars a year in value from monoclonal antibodies alone.
But that’s far from the end of the story. You know what else is made with mammalian cell culture?
Gene therapy
the viral vectors that deliver many gene therapies to cells are grown in mammalian cell culture
Cell therapies
including the immune cell cancer therapies such as CAR-T that have recently transformed oncology
Stem cell therapies
Lab-grown meat
All of these are small markets today. Gene therapies, T-cell therapies, and stem cell therapies are each under $10B/year, and lab-grown meat hasn’t yet been commercialized. But these are the biotechnologies of the near future. They’re growing rapidly, and they have the potential to be transformative for nearly every disease and the way the planet feeds itself.
As an aside on cultured meat, there’s a credible techno-economic analysis arguing that it’s never going to be cost-competitive with animal meat. But the calculations extrapolate from the costs of mammalian cell culture in the current pharmaceutical industry, and cell culture costs are most of the total manufacturing cost. It’s probably fairer to say that unless cell culture gets radically cheaper and more efficient, cultured meat isn’t going to be commercially viable.
When it comes to cell therapies, current manufacturing yields are even lower and costs even higher than in antibody production. Only 28-38% of autologous (patient-derived) cell therapy batches are usable, and a single dose of a cell therapy, whether autologous or allogeneic (donor-derived) costs thousands of dollars to manufacture.3 4 5.
Even these low yields may be overestimates in practice, since manufacturers of cancer T-cell therapies have struggled with quality control problems and have provided defective treatments to many patients. In 2019, Novartis was found to have provided 28% of cancer patients with defective doses of their T-cell therapy Kymriah, containing fewer than 80% viable cells.6
The bottom line is that, while cell therapies are as yet a fairly small market with few approved drugs in the category, it is even more crucial for cell therapies than for antibody therapies that the technology for cell culture improves to increase the yield of viable cells and reduce manufacturing costs.
A Little Rant On Yield
“Yield”, to a manufacturing process engineer, represents the amount of usable end product produced per unit of raw materials. It can be given as a percentage — what % of the stuff produced from a given quantity of raw materials is good quality enough to sell?
To an investor or economist, “yield” is a somewhat different but related quantity: it’s the amount of usable product created per dollar spent.
So you can increase economic “yield” without changing the physical manufacturing process, if you increase something like uptime (running your plant more hours per year), or reduce labor/equipment/materials costs, or something like that.
You will increase both manufacturing yield and economic yield if you make the production process more efficient, so that you can get more output from the same amount of raw materials (and energy).
You will also increase both manufacturing yield and economic yield if you reduce the rate of defects and failures in the production process. The less product you have to throw out as defective, the more you get for your dollar or unit of raw materials.
Simple, right?
Yet manufacturing companies often think of “reducing defects” (or maintaining quality control) as a separate magisterium from “improving manufacturing productivity,” and think of both as separate from “growing revenue.” Quality control is viewed as a cost center, and tends to have a very cautious and conservative departmental culture. If new manufacturing technology gets pigeonholed as “quality control” it’s likely to be deprioritized, viewed with suspicion, and given a small budget.
Likewise, if radical technological improvements in manufacturing yield get pigeonholed as “cutting manufacturing costs” they’re likely to be underappreciated compared to their real impact. Yes, increasing yield cuts costs — but a significant enough increase in yield can also open new markets and provide a multiplier on revenue.
Think of the example of cultured meat — there’s virtually no market for cultured meat at the current cost of production, but once it drops below the cost of producing animal meat, it’s worth trillions per year.
How many more patients per year would receive cell therapies if a course of therapy didn’t cost them six figures?
From a certain point of view, the high cost and poor reliability of mammalian cell culture is a “quality control” issue, in that the problem is that too many cells are dying or nonviable. But the stereotype of “quality control” as fundamentally a conservative, defensive specialty doesn’t apply when “failure” rates are this high. Here, quality improvements would mean massive growth and transformation for the whole industry.
How Can We Raise Mammalian Cell Culture Yields? Adaptive Control.
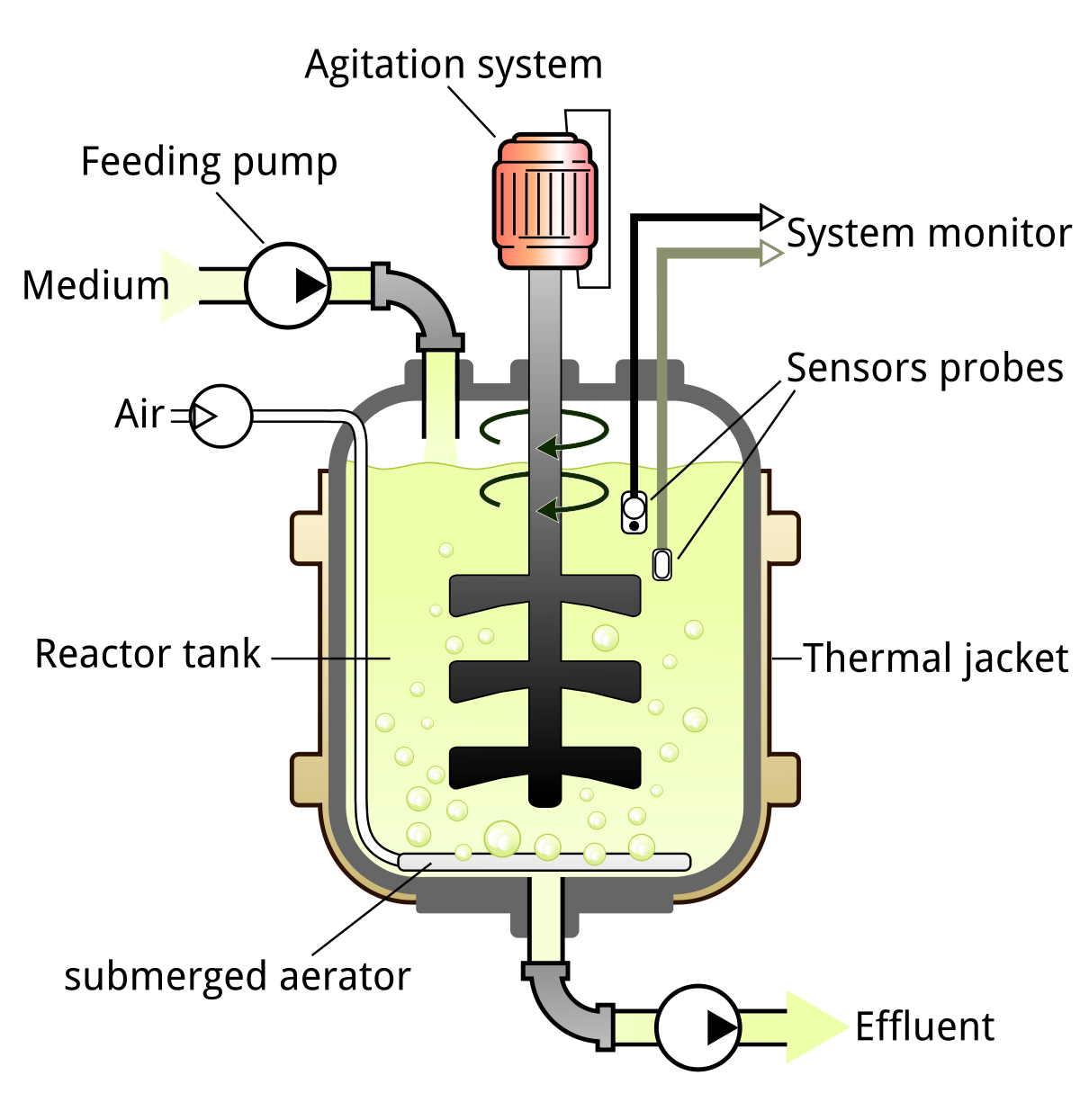
Industrial cell culture happens in a bioreactor like this:
Most of what goes on in the reactor is under automated control. The reactor is kept at a fixed, cell-friendly temperature; medium is pumped in at a controlled rate, air is bubbled in likewise, pH is monitored and kept at a target level, etc.
In batch culture, medium is put in the reactor at the beginning of the batch, and the cells keep growing until the nutrients run out. In fed-batch culture, you monitor the concentration of nutrients and replace them when they run out.
In perfusion culture, the most advanced method and the one used by most pharmaceutical manufacturers for antibody production, you continuously add nutrient-containing medium and continuously remove waste and harvest product. Perfusion produces the highest yields, as measured by cell density (per volume) and cell viability (as a % of cells).
This process, like many industrial chemical processes, is under automated control — sensors continuously check the temperature, gas levels, glucose and lactic acid concentrations, etc, and adjust actuators automatically to keep the bioreactor running at the target values. If glucose gets to be too high, less glucose is pumped in; if it’s too low, more is pumped in.
But cell culture, as practiced today, is not under adaptive control.
The target levels for things like temperature or pH or glucose concentration are fixed, hard-coded. Usually the target values programmed into the bioreactor are determined experimentally, through laborious trial and error. (And remember, the settings that worked in the lab won’t necessarily work in the factory — all the experimentation has to be redone for each successive scale-up of production!)
Research has shown you get much higher yields under adaptive control — if you allow the target levels for bioreactor settings to adjust as the culture grows or in response to measured conditions in the cell culture. The ideal growing conditions for a low-density culture are different from the ideal conditions for a high-density culture after cells have been growing for a while.
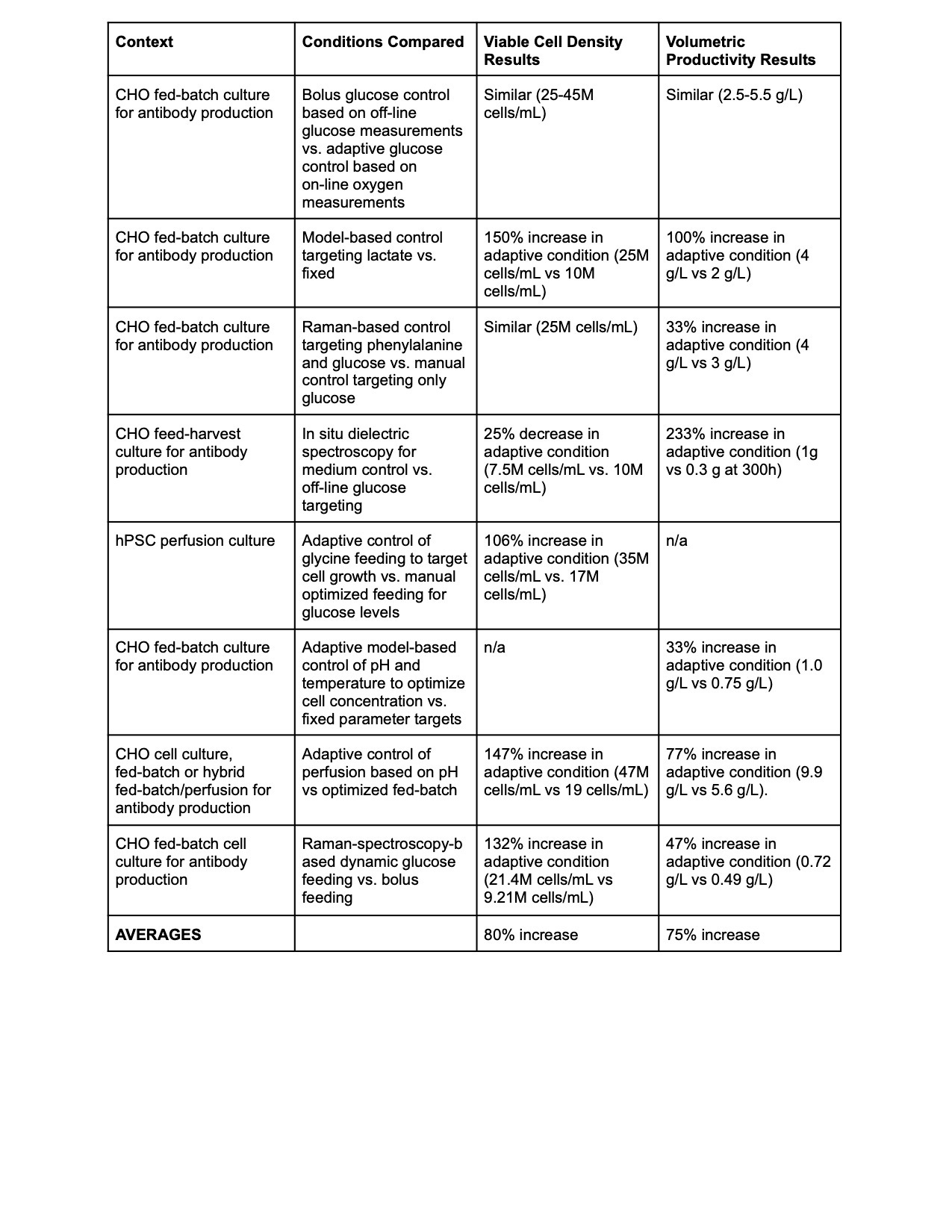
Aggregating 8 recent papers (all the relevant ones I could find published since 2017) on different kinds of dynamic control of cell culture finds that dynamic control of even a single process parameter nearly doubles yields.7 8 9 10 1112 13 14
Now, one caveat to keep in mind is that the real state of the art as practiced in the pharmaceutical industry is always somewhat beyond what’s published in papers. For one important example, academic reasearchers on cell culture use 1970’s-era culture medium with standard, published ingredients. Pharma companies use more modern, highly optimized culture media whose formulas are closely guarded trade secrets.
So we should expect the baseline yields in the “real world” to be better than what’s published in journals, and the “real world” improvements due to dynamic control to be correspondingly smaller.
But there’s also reasons to believe the 75%-80% yield improvements reported in the papers are underestimates.
First, notice that different experiments use adaptive control for different parameters — some control pH, some control glucose, some control temperature, some control specific amino acids included in the nutrient medium like glycine or phenylalanine. So dynamically controlling all nutrient inputs and reactor parameters at once could plausibly more than double yields.
Secondly, all these papers use mathematical models of cell growth and metabolism to estimate the effect of a given change to cell culture conditions (temperature or pH adjustments, glucose input rate, amino acid input rate) on measurable values (glucose concentration, lactic acid concentration, amino acid concentration, temperature or pH). Then, “adaptive control” as practiced in these experiments computes the desired adjustment by applying the inverse function to the measurements.
In reality, cell growth is highly complex, and these mathematical models are not necessarily good approximations to what’s actually going on in the reactor. Cell culture is one of the situations where model-free, nonparametric machine learning might very well outperform closed-form mathematical models. “Learning” something like the right nutrient feed rate to hit a given lactate concentration might work better with a big neural network and a large volume of experimental data than the traditional, model-based control-theory way.
Thirdly, with these model-based adaptive control methods, researchers are still often targeting a “hard-coded”, static, experimentally-determined number like the “ideal” pH or lactate or glucose concentration. This is only a proxy for the numbers we really care about — cell density and cell viability.
If the “ideal” target derived in the lab is actually not the optimal level for cell growth in industrial practice, as seems plausible, then adaptive control of cell culture to hit that “ideal” target isn’t the best we can do to improve yields, and there’s still room to outperform even the significant yield improvement in the literature.
Full Adaptive Control: The Dream
What you’d really like to do is measure how many cells are in the reactor and how healthy they are, continuously and without disturbing the culture, and then optimize reactor conditions to maximize cell density and/or viability.
Your machine learning model would then be trained on the prediction problem “what will measured cell density/viability be, given current measurements of temperature, pH, O2, lactate, glucose, etc, and given a potential change in nutrient flow rates, temperature, pH, gas flow rates, etc?”
Then your “smart bioreactor” would make the adjustments that maximize the ML-predicted cell density and/or viability.
This isn’t actually that big a model to train. There are probably well under 50 types of sensor measurement and reactor-controlled parameters worth including, all of which are producing sensor data or logging actuator behavior at perhaps a few hertz. Language or video-based machine learning models are much higher-dimensional and need correspondingly vast quantities of training data.
But how do you measure the cells in a reactor in real-time and noninvasively?
In-Line Cell Density & Viability Measurement
One method for detecting how many cells are in your bioreactor is dielectric spectroscopy. Living cells (with unruptured cell membranes) have a different electrical impedance than the surrounding culture medium, and so permittivity probes placed in the culture medium can measure cell concentrations in real time.15 Other details of cell physiology such as cell size, growth rate, or cellular stress may also be detectable with impedance spectroscopy. Researchers at Biogen recently implemented an impedance-spectroscopy system in mammalian cell culture and found it could be used to optimize16 nutrient feed rates in a batch-fed process, resulting in a 21% increase in cell titer and reducing the risk of process failure due to underfeeding.
Similarly, NIR (near infrared) sensors can be used to measure cell density, taking advantage of the fact that cells have different absorption spectra than the surrounding medium. This method, unfortunately, does not distinguish between live and dead cells.
Another potential way to measure cell density and viability in real time is simply to continuously feed a sample from the bioreactor through a microfluidic device which can be monitored optically with a digital microscope. Once you have microscopic images of a small volume of the cell culture, automatic image recognition can count cells, and perhaps measure some physiologically relevant morphological features such as average cell size or number of currently-dividing cells.
In-situ microscopy, in which a microscope is directly attached to a port on the bioreactor and image processing is used to analyze microscopic images of cells, has been known since the 1990s as a potential method for quantifying cell density in culture17 but it hasn’t seen widespread industrial adoption due to its high cost.18 That might be worth revisiting today, now that there’s been significant progress in both microfluidics and image recognition algorithms.
There are a lot of options for nondestructive in-line cell culture monitoring that are currently sitting at the boundary between research and applied use. I think it’s pretty likely that the remaining kinks can be worked out of at least one of these technologies, if manufacturers can be convinced it’s worth it to invest in integrating new sensors into their cell culture processes.
Who’s Working on This?
First of all, it’s a good bet that the cell culture development department at every major pharma company or biologics-focused contract manufacturing company is at least starting to experiment with adaptive control of mammalian cell culture and trying out some new in-line sensors.
On the other hand, usually these cell culture dev departments don’t have machine learning expertise, so that part of the picture is still likely neglected.
What’s going on in the startup world with attempts to make cell culture more efficient?
To my knowledge, there are no startups that specialize in adaptive process control for improving yield and scalability of cell culture.
There are a fair number of adjacent technological applications, though, that people have built companies around.
Massively Parallel Cell Culture Manufacturing
Neuroscientist-turned-entrepreneur Gaurav Venkataraman has a very early-stage startup, Trisk Bio, whose initial angle of attack is brilliantly simple. Scaling up cell culture from benchtop to factory is unreliable? So just make tons of benchtop-sized bioreactors for your factory.
Parallelizing small-volume cell culture doesn’t itself improve manufacturing yield relative to a large-volume cell culture production process, but it does reduce the R&D cost of manufacturing a new cell-culture-based therapeutic.
Parallelized, modular cell culture is also a very powerful experimental platform for comparing different culture conditions and measuring their effect on yields. It’s a force multiplier on any other research program for improving cell culture yields.
Cell biologist Michael Todhunter recently received an ACX Grant of $40,000 for building a robotically automated system to optimize cell culture medium composition by testing dozens of cell culture samples in parallel under different media conditions. This may be a good opportunity for additional grant funding if the ACX grant isn’t sufficient to produce a prototype.
Automating Culture for Cell Therapies
There’s a lot of work in the cell and gene therapy space on automating the cell culture process. Companies like Avrobio, Ori Biotech, and Cellares are producing robotic, self-contained devices that can genetically modify and expand cells derived from patients or donors. The leading manufacturers of cell culture bioreactors like Miltenyi and Lonza have also come out with (relatively) automated devices designed for cell therapy.
Automating cell culture (where it’s not already automated) can be expected to increase economic yields by reducing labor costs and turnaround times, but by itself it won’t necessarily increase manufacturing yields (the density of viable genetically-modified cells produced per sample of donated cells.).
And there’s a lot of automation already in more-established mammalian cell manufacturing processes like those used to make antibody therapies.
Automated Manufacturing for Stem Cell Therapies
There’s also a fair number of startups working on automating the process of generating even a small quantity of clinical-grade therapeutics derived from pluripotent stem cells.
Stem cells are even more delicate and difficult to manufacture than most mammalian cells; the cost per dose of a typical stem cell therapy is over $1M.
Companies like TreeFrog Therapeutics boast of “scale-up” and “mass production” of stem cell therapeutics…while displaying a photo of a benchtop device.
This is ultra-cool but it’s not industrial scale.
Cell Line Discovery
There’s also a world of startups working on automating an earlier stage of the cell culture manufacturing process — developing lines of cells with desirable properties for therapeutics.
If you’re making antibodies, you want cells that produce a lot of your favorite antibody; if you’re making viral vectors, you want cells that enable lots of virus proliferation; if you’re making cancer immunotherapies, you want cells that attack cancer cells and don’t attack anything else. No matter what you’re making, you want cells that grow well in culture and don’t die.
All of this means selecting a line of cells with the right genes and the right expression profile for the application you want — either with old-fashioned selective breeding, or with genetic engineering, or a combination of the two. And, as always when you want to find a needle in a haystack, automation and massive parallelization are your friends.
Hence, cell engineering startups like 64x, Asimov, bit.bio, and more.
The right cell line can certainly increase mammalian cell culture yield, and accelerating cell line discovery can also expand the number of cell-derived therapies that make it into the clinic.
But it’s a separate problem from optimizing the conditions of cell culture in known cell lines, which is the main topic of this post.
The bottom line is that, despite a buzzy, crowded field of startups and established companies working on biomanufacturing technology, improving the efficiency of mammalian cell culture itself is still a wide-open opportunity.
Bunnak, Phumthep, et al. "Life‐cycle and cost of goods assessment of fed‐batch and perfusion‐based manufacturing processes for mAbs." Biotechnology progress 32.5 (2016): 1324-1335.
Klutz, Stephan, et al. "Cost evaluation of antibody production processes in different operation modes." Chemical Engineering Science 141 (2016): 63-74.
Jenkins, Michael J., and Suzanne S. Farid. "Cost-effective bioprocess design for the manufacture of allogeneic CAR-T cell therapies using a decisional tool with multi-attribute decision-making analysis." Biochemical engineering journal 137 (2018): 192-204.
Chilima, Tania D. Pereira, Fabien Moncaubeig, and Suzanne S. Farid. "Impact of allogeneic stem cell manufacturing decisions on cost of goods, process robustness and reimbursement." Biochemical engineering journal 137 (2018): 132-151.
Goldrick, Stephen, et al. "On‐line control of glucose concentration in high‐yielding mammalian cell cultures enabled through oxygen transfer rate measurements." Biotechnology journal 13.4 (2018): 1700607.
Schmitt, John, et al. "Forecasting and control of lactate bifurcation in Chinese hamster ovary cell culture processes." Biotechnology and bioengineering 116.9 (2019): 2223-2235.
Webster, Thaddaeus A., et al. "Feedback control of two supplemental feeds during fed-batch culture on a platform process using inline Raman models for glucose and phenylalanine concentration." Bioprocess and Biosystems Engineering 44.1 (2021): 127-140.
Li, Meng‐Yao, et al. "Control of IgG glycosylation by in situ and real‐time estimation of specific growth rate of CHO cells cultured in bioreactor." Biotechnology and bioengineering 116.5 (2019): 985-993.
Manstein, Felix, et al. "High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling." Stem cells translational medicine (2021).
Paul, Katrin, Vignesh Rajamanickam, and Christoph Herwig. "Model-based optimization of temperature and pH shift to increase volumetric productivity of a Chinese hamster ovary fed-batch process." Journal of bioscience and bioengineering 128.6 (2019): 710-715.
Hiller, Gregory W., et al. "Cell‐controlled hybrid perfusion fed‐batch CHO cell process provides significant productivity improvement over conventional fed‐batch cultures." Biotechnology and bioengineering 114.7 (2017): 1438-1447.
Domján, Júlia, et al. "Raman‐based dynamic feeding strategies using real‐time glucose concentration monitoring system during adalimumab producing CHO cell cultivation." Biotechnology Progress (2020): e3052.
Flores-Cosío, G., et al. "Application of dielectric spectroscopy to unravel the physiological state of microorganisms: Current state, prospects and limits." Applied Microbiology and Biotechnology 104.14 (2020): 6101-6113.
Moore, Brandon, Ryan Sanford, and An Zhang. "Case study: the characterization and implementation of dielectric spectroscopy (biocapacitance) for process control in a commercial GMP CHO manufacturing process." Biotechnology Progress 35.3 (2019): e2782.
Bittner, C., G. Wehnert, and T. Scheper. "In situ microscopy for on‐line determination of biomass." Biotechnology and bioengineering 60.1 (1998): 24-35.
Reardon, Kenneth F. "Practical monitoring technologies for cells and substrates in biomanufacturing." Current Opinion in Biotechnology 71 (2021): 225-230.