Marine Carbon Dioxide Removal
Should we be taking CO2 out of the atmosphere and putting it in the oceans?
Yesterday, I went to a panel discussion on marine carbon dioxide removal, at New York Climate Week.
It was kind of a chance invitation. I’m not a “climate person” in the slightest; my last exposure to earth science was in the eighth grade. But I’m no stranger to going to conferences where I’m the only non-expert in the room. It’s always delightful to be among dressed-up, civilized people drinking free coffee and learning something new. (And, for me, it’s a rare treat to be at an event that’s 50% women.)
There was a lot of talk about the need for outreach beyond the “MCDR community”, and to communicate to laymen what MCDR is all about and why it’s a good idea. Given that I’d never heard of it before, I have to agree that it’s under-publicized.
Climate-world is weird, by my standards. It’s inherently highly collaborative. You need these big consortiums of researchers and partnerships with governments in order to even get the necessary data and put these giant models together. Serious climate change mitigation strategies are engineering megaprojects, which are also inherently collaborative and intersect heavily with public policy. It’s hard to do anything in climate — or even understand it — without leaning heavily on other people’s work, and playing nice with lots and lots of people and large institutions. This is part of why I’ve been reluctant to touch it. It’s just not a loner-friendly field.1
But we do, after all, all live here on Earth. Making sense of climate change, and the proposals to do something about it, is important. Even if the best I can do at the moment relies heavily on trusting claims I can’t check to my usual degree of rigor.
(m)CDR Basics
So, what is marine carbon dioxide removal?
Basically what it sounds like. A subcategory of geoengineering, involving taking carbon dioxide out of the air and into the ocean.
Why would we do this?
The “climate math”, as they like to say, is unequivocal: even if the world steeply reduces carbon dioxide emissions, a fair amount of global warming and disruptive change is bound to happen. It’s impossible to stabilize the climate unless we also do some sort of geoengineering to reverse the greenhouse effect.
Taking CO2 out of the atmosphere is one of the main categories of geoengineering2 being considered today.
Why do CO2 removal in the ocean? Well, the ocean is big. It covers 72% of the Earth’s surface. Also, the ocean is already a major CO2 buffer, storing about 25% of man-made CO2 emissions per year (or about 9 gigatons/year). Increasing that storage rate, if it’s feasible, is an opportunity for making a major impact on CO2 levels.
There are, currently, about 3300 gigatons of CO2 in the atmosphere.3
IPCC’s target4 for carbon dioxide removal is that we’d need to take 2-13 gigatons of CO2 per year out of the atmosphere — 260-1030 gigatons total — to confine global warming to 1.5 degrees by 2100.5 The National Academy of Sciences estimates we’d need 10 Gt/year by 2050 and 20 Gt/year by 2100.6
In other words, we need to remove roughly 7-40% of the CO2 in the atmosphere, over the course of a century. That’s huge. Any method needs to be extraordinarily cheap and scalable to be viable.
But on the positive side, you might need only a 10% increase in the ocean’s carbon sequestration rate by 2050 to meet those targets!
The only widely deployed carbon dioxide removal (CDR) method today is reforestation. Plants, of course, “breathe” carbon dioxide; forests already sequester a comparable amount of CO2 to oceans, at 7.6 gigatons/year.7 And the nice thing about planting trees is that it’s not a risky new technology. But, unfortunately, land that you plant trees on is land you can’t use for farms, factories, or towns.8 Reforestation ultimately means governments, usually in the poorer countries that still have big forests, imposing economic costs on their citizens. The space requirements for reforestation are huge; achieving the entire CDR target through reforestation alone would require turning an area the size of mainland China into forest.9
Ideally we’d like a CDR method that’s more economically efficient.10
We’d like to react carbon dioxide with some substance (a “sorbent”) that’s non-toxic, extremely abundant and cheap, and stores the carbon (more-or-less) permanently at a modest energy cost, and doesn’t require reserving large areas of economically productive land.
Fortunately, there are lots of common, cheap, non-toxic minerals that react with CO2. Olivine is one; limestone is another. Grind ‘em fine (to increase surface area) and spread ‘em out somewhere where they don’t interfere with human activity or natural ecosystems (barren land or the ocean floor). In fact, this strategy can be beneficial to aquatic ecosystems, because dissolved alkaline substances can counteract the acidification caused by excessive dissolved CO2, which has caused mass die-offs of aquatic organisms, including coral reefs, in recent years.
You could also, in principle, return to “reforestation” in a less land-hungry way; you could either create plants that are much more efficient at sequestering carbon, or you could grow your plants at sea (via increasing algae or seaweed populations) where they won’t compete with human activities. These approaches tend to be more problematic and/or speculative than the mineral-based techniques, but we’ll consider that in more detail further on.
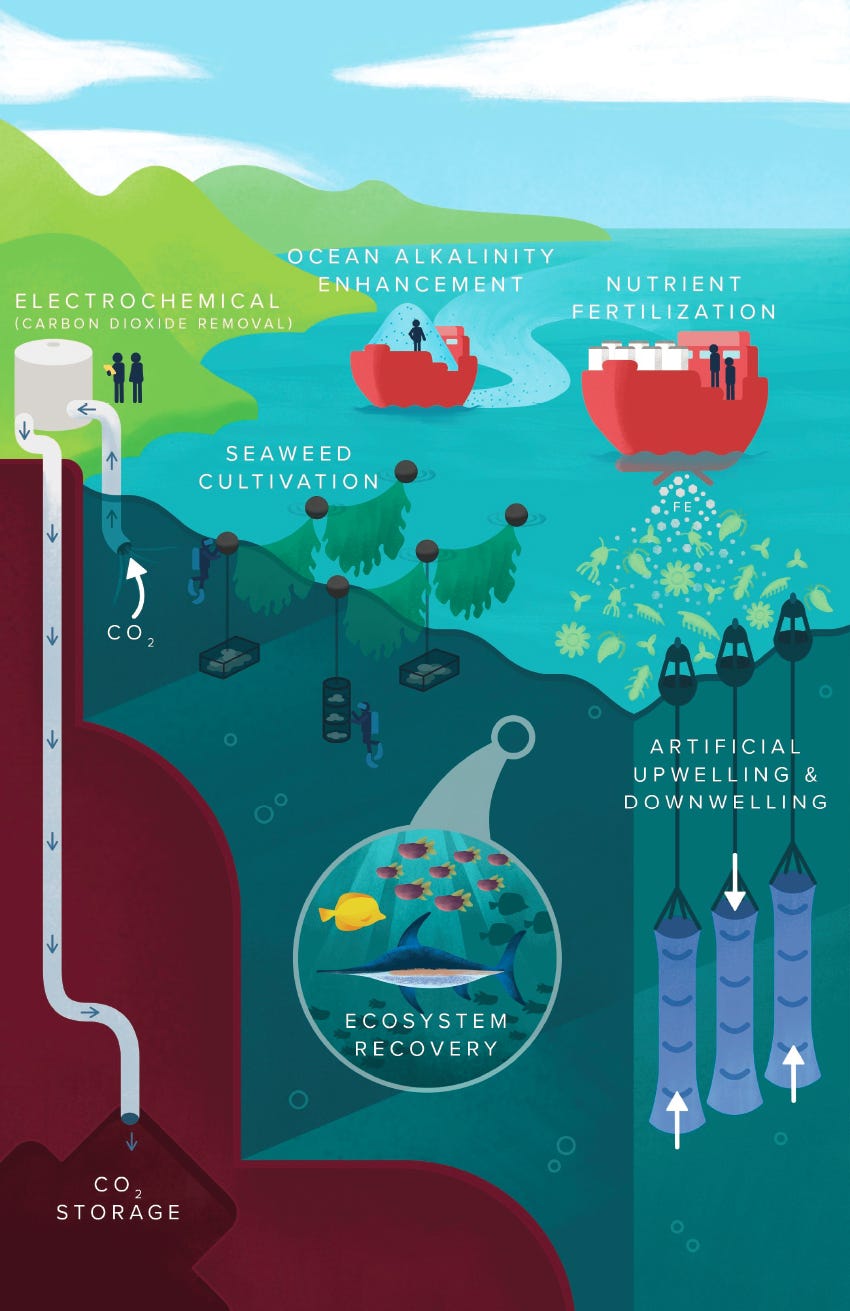
mCDR Methods
The National Academies of Science published a long, informative report on MCDR in 2021, summarizing multiple proposed methods visualized above.
Four of these (ecosystem recovery, artificial upwelling & downwelling, nutrient fertilization, and seaweed cultivation) are tactics to grow more aquatic plants for photosynthesis (and the resulting natural carbon sequestration). Nutrient fertilization and upwelling deeper, nutrient-rich water are both ways to cause more algae growth; seaweed farms (obviously) grow seaweed; and restoring damaged ecosystems like kelp forests also creates plant biomass.
The other two (electrochemical methods and ocean alkalinity enhancement) are both about dumping compounds in the ocean that increase the amount of CO2 it can hold by changing the water’s pH. (CO2 dissolves more in more alkaline environments.)
Seawater Chemistry
The basic chemical equation you need to understand ocean CO2 is:
CO2 (aq) + H2O ↔ H2CO3
Dissolved carbon dioxide and water make carbonic acid, the mild acid found in seltzer.
It’s an acid because it can drop either one or two hydrogen ions, and pH is measured by the (inverse) concentration of hydrogen ions in a solution.
Seawater is, of course, full of salt, and salt is a buffer; its chloride ions sop up hydrogen ions, keeping the ocean from getting as acidic as freshwater with dissolved CO2 would.
Now, we’d like to have more of the right-hand side of the above chemical equation and less of the left. The more CO2 you put in the water, the more you “push” the reaction in the forward direction, producing carbonic acid. That’s why CO2 emissions cause ocean acidification.
If you put alkaline compounds or carbonates in the water, this lowers dissolved CO2. The lower concentration of CO2 in the water makes it take in more CO2 from the air.
The ocean is big. It contains about 38,000 gigatons of (inorganic) dissolved carbon — a stock that dwarfs the much smaller flows of anthropogenic CO2 emissions, and even exceeds (by 44x!) the amount of carbon in the atmosphere and (by a smaller margin) the carbon stored in soil, minerals, and hydrocarbons in the earth’s crust. There is far more than enough room for all anthropogenic CO2 to be stored in the ocean.
Growing More Marine Plants
We absolutely can grow more marine plants.
In fact, it’s dirt cheap; (in)famously, you can cause giant algal blooms by dropping iron filings in the ocean. Oceanographer John Martin, in 1988, said “Give me a half tanker of iron, and I will give you an ice age.”
This has the potential to increase carbon sequestration by a gigaton/year or more.11 And it would cost less than a billion dollars to do the whole thing!
One problem is that people hate it.
Back in 2012 a controversial businessman, Russ George, dumped 100 tons of iron off the west coast of Canada, to widespread environmentalist outrage. He was dubbed a “rogue actor” and accused of violating international laws (they were actually nonbinding resolutions).
But a bit of bad PR doesn’t spell doom for nutrient fertilization by itself.
A more serious problem is that some algal blooms are toxic. If you just put iron in the water, you don’t control which species will grow; so far, experiments have mostly resulted in non-toxic algae growth, but the risk is concerning.
Another problem, which is common to all plant-based approaches, not just nutrient fertilization, is that the CO2 will just be released back into the atmosphere when the plants decay, unless you sequester them somehow (sinking to the seafloor?)
Seaweed cultivation, unlike nutrient fertilization or upwelling/downwelling, is definitely nontoxic; but it’s more difficult to scale. (The NAS estimates that it can only get up to 0.1 Gt/year).12
Decreasing Seawater CO2
Silicates and carbonates, dissolved in seawater, add alkalinity, “locking” carbon into carbonate and bicarbonate ions rather than dissolved CO2. This increases the amount of CO2 that leaves the atmosphere to dissolve in the ocean — and, in turn, some of that CO2 gets “locked up” as carbonate/bicarbonate.
To do this at scale, you need a lot of ground up rocks, and you need energy to grind them to powder. But the rocks themselves are incredibly abundant. Olivine, limestone, basalt — this is the stuff the Earth’s crust is made of.
The NAE estimates this can get up to 1-4 Gt/year of carbon out of the atmosphere — quite substantial, even better than nutrient fertilization, albeit at a higher cost per gigaton.13
Electrochemical methods are fundamentally similar, except that instead of placing alkaline substances in the water, they create alkalinity at the cathode of an electrolysis reaction (and acidity at the anode) by running a current. The acidified water around the anode is full of CO2, which you can vent and store underground; the alkalinified water around the cathode you can put back in the ocean, where it’ll suck CO2 out of the atmosphere.
Electrolysis is energy- and capital-intensive, and produces some toxic waste products (like chlorine) though they can also be saved and sold commercially if you can recover it effectively. On the whole though, this is one of the pricier methods (at best $150/t CO2).
Who’s Working On It?
A nonprofit called C-Worthy which builds software models and runs experiments on the effectiveness of mCDR has produced a handy mCDR ecosystem map, including funders, providers of infrastructure and MRV (monitoring/reporting/verification) tools, and mCDR providers themselves.
There’s a bunch of startups that sink algae or seaweed, as well as one that provides electrochemical alkalinity enhancement and a few that provide mineral-based alkalinity enhancement (one olivine, one magnesium hydroxide).
Right now, the greatest need is for well-monitored pilot projects so we can observe how effective they are (and detect any harmful environmental side effects).
Where Will The Money Come From?
Ultimately, if we want to get up to 10 Gt/year of CDR, stacking multiple methods, the price tag will be hundreds of billions of dollars a year at least.
The world is already spending comparable amounts on climate change mitigation and adaptation, but almost all of that is emissions reduction, so adding on CDR would be a substantial cost increase.
And, unlike electric cars or solar power, there isn’t usually a straightforward way to make mCDR profitable (though algal blooms and ocean deacidification can result in bigger fishing catches, so maybe there could be a viable business selling ocean restoration for fisheries?). The benefits of limiting global warming are distributed globally; it’s a classic public good, not excludable or rivalrous.
Ultimately, if CDR is to happen, the costs will be imposed by governments on their citizens — either through tax-funded projects, or through regulatory requirements or some carbon-tax-like mechanism.14
In the short run, there’s private funding sources — philanthropy, venture capital, advance market commitments like Frontier Climate’s $1B fund, voluntary net-zero targets by corporations and voluntary carbon-credit markets — but those aren’t going to take us all the way there, and they generally only exist in anticipation of a future government effort.
To date, not a lot has been spent on mCDR: probably not more than $100M, total.
What I mostly heard the conference people emphasizing was that we need more pilot experiments, more monitoring, and more coordination with governments, in order to get clear regulatory guidance and collaboration with national/international climate monitoring organizations (like NOAA in the US). Right now, mCDR needs money, but even more importantly it needs permits to run experiments.
Takeaways
If you take the IPCC’s predictions as a given, we cannot have limited global warming without geoengineering. Emissions reductions are necessary but not sufficient.
Taking the National Academy of Science’s estimates as a given, carbon dioxide removal from the atmosphere has the potential to remove at least half of all human-added CO2 from the atmosphere — and marine CDR can be a substantial fraction, if not the majority, of all viable CDR methods. In particular, the cheaper and more scalable methods (mineral-based alkalinity enhancement and algae nutrient fertilization) are ocean-based.
So yeah: mCDR is a Thing, and should be a lot better known. It’s one of the major viable avenues for countering climate change.
and while there are plenty of economically viable climate-relevant technologies, including solar and electric vehicles, if you don’t like regulation or subsidies you are going to have to do a lot of awkward smiling and nodding.
another major category is reducing the amount of sunlight that hits the earth, or reflecting more of it into space
https://gml.noaa.gov/ccgg/trends/
https://www.ipcc.ch/sr15/chapter/chapter-2/
I’m taking the IPCC’s targets for granted, for the purposes of this post. I’m also not going to discuss whether it’s “worth it” to avoid 1.5-degree warming. I’m not knowledgeable enough to second-guess climate and economic models. I’m more focused on, “given that you want to achieve the standard IPCC Net Zero/<1.5-degree-warming goal, how can you do it?”
https://nap.nationalacademies.org/read/18805/chapter/1
https://www.wri.org/insights/forests-absorb-twice-much-carbon-they-emit-each-year
Planting trees in cities is really great; it reduces local temperatures, making summer heat waves less intense, and it generally makes for a more pleasant urban environment. So in a local sense trees don’t “compete” with homes and businesses. But urban tree-planting is way too small-scale to make a dent in global atmospheric CO2.
https://www.science.org/doi/10.1126/science.aax0848
We care about economic efficiency more than chemical efficiency, for the purpose of achieving climate-stabilization goals humanely. Trees aren’t very chemically efficient at converting CO2 to other compounds, and you can get much better efficiency with industrial processes. But that doesn’t help much if the industrial processes are too expensive to be practical at massive scale, and many carbon-capture methods are, usually because they are highly energy-intensive.
https://nap.nationalacademies.org/read/26278/chapter/5#88
https://nap.nationalacademies.org/read/26278/chapter/7#132
https://nap.nationalacademies.org/read/26278/chapter/9#195
Personally, I’m not thrilled with that, but it’s important to be honest about the political/economic reality.





Minor point: “Seawater is, of course, full of salt, and salt is a buffer; its chloride ions sop up hydrogen ions, keeping the ocean from getting as acidic as freshwater with dissolved CO2 would”
This is not true. Chloride ions don’t act as a buffer, but other organic ions (carbonate and bicarbonate , mainly) would.
You said that there’s 3300 Gt of CO2 in the atmosphere and 38000 Gt of carbon in the ocean but said that the stock of carbon in the ocean dwarfs the amount in the atmosphere by 44x - was that an error or am I missing something? Seems more like 11.5x