Minicircle is a startup claiming to have produced “the world’s first reversible gene therapy platform.”
They have a clinic in Prospera , the libertarian/crypto special economic zone in Honduras, where you can get their first experimental gene therapy: a protein called follistatin, which is supposed to increase muscle growth.
Follistatin is a natural inhibitor of myostatin, the protein that puts the brakes on muscle growth. Animals deficient in myostatin have a distinctive “double-muscled” build:
So, naturally, people have long wondered whether genetically or chemically inhibiting myostatin could help people increase muscle mass.
It’s a natural thing to be excited about, and (as your friendly neighborhood libertarian transhumanist) I’m drawn to the idea of a Prospera-hosted biotech company making people superhumanly jacked.
The question, of course, is whether it works.
That’s what this post is about.
I have no affiliation with the Minicircle people and have never met them — this post is all drawn from public information.
And their public information about their own therapy is pretty sparse. Their “trial” of 44 people is not a controlled trial.
That means their results so far are not clearly distinguishable from noise — for instance, it would not at all be surprising for people newly enthusiastic about their muscle-building gene therapy to start a new exercise regimen and gain 2 pounds of muscle over 3 months from that alone.
They’re going straight for the media attention (with a high-profile invitation to Bryan Johnson) rather than the slow-and-steady clinical trial approach, but I don’t really care about that one way or another. The world needs a diversity of approaches, including higher-risk, speedier options than traditional FDA clinical trials.
The question that’s actually interesting to me is plausibility. Before any real results are in, how much should we expect that a follistatin plasmid therapy might work? And how risky should we expect it to be?
For that, I don’t want to look at Minicircle at all, I want to look at comparable approaches.
How effective is follistatin, or myostatin inhibition more broadly, for muscle gain? (and what kinds of safety risks are there)
How plausibly effective (and safe) are minicircle plasmids as a means of gene therapy delivery?
Follistatin
The animal literature is extremely consistent that follistatin, whether administered as a protein, an AAV gene therapy, or transgenically modified from birth for higher expression, increases muscle mass and strength and decreases fat mass.
Follistatin is very real.
In humans, of course, you’re not going to find anyone doing a clinical trial of follistatin’s effects on body composition in healthy people, because that’s not a medical indication. Most of the trials are for muscular dystrophy and other muscle disorders.
In that context, there’s a single (possibly out-of-business) startup using follistatin gene therapy in clinical trials, and results look promising.
Mice
A follistatin AAV gene therapy increased muscle size and strength, including in muscles remote from the injection site.1
Another follistatin AAV gene therapy using the 288-aa isoform doubled muscle mass and produced a 40% increase in force capacity within 28 days of treatment. This growth was from muscle fiber hypertrophy, not an increase in the number of muscle fibers. In fact, the muscle growth effect even works in myostatin-null mice, so follistatin-288-aa has some effects independent of the myostatin inhibition.2
AAV overexpression of follistatin-288 increases muscle mass as well as glucose uptake into muscles, and blocks fat gain for mice on high-fat diets.3
Transgenic mice overexpressing follistatin had 70% more muscle mass than wild-type.4
An engineered follistatin-288-Fc fusion protein injected into muscle makes that muscle, but not others, grow in mice.5
AAV follistatin overexpression in mice increased muscle mass (by 50-100% depending on muscle) as well as reducing fat mass (by 80%).6
Daily subcutaneous injection with recombinant human follistatin-288 reduced weight gain in mice over a 13-week period, increasing lean mass 10% over the 13 weeks and reducing fat mass 53%. The treated mice increased their ambulatory activity but, interestingly, had lower overall energy expenditure (measured by heat) and lower VO2 (oxygen intake).7
A 2021 study of a cytomegalovirus gene therapy containing follistatin8, administered monthly, extended mouse median lifespan 32.5%, increased body weight and improved coordination in old age (as measured by speed at a beam-crossing test).
Rats
Rats injected with plasmids to overexpress follistatin increased muscle mass in rats. This worked just as well in myostatin-deficient animals, so it’s not just a myostatin inhibition effect.9
Fish
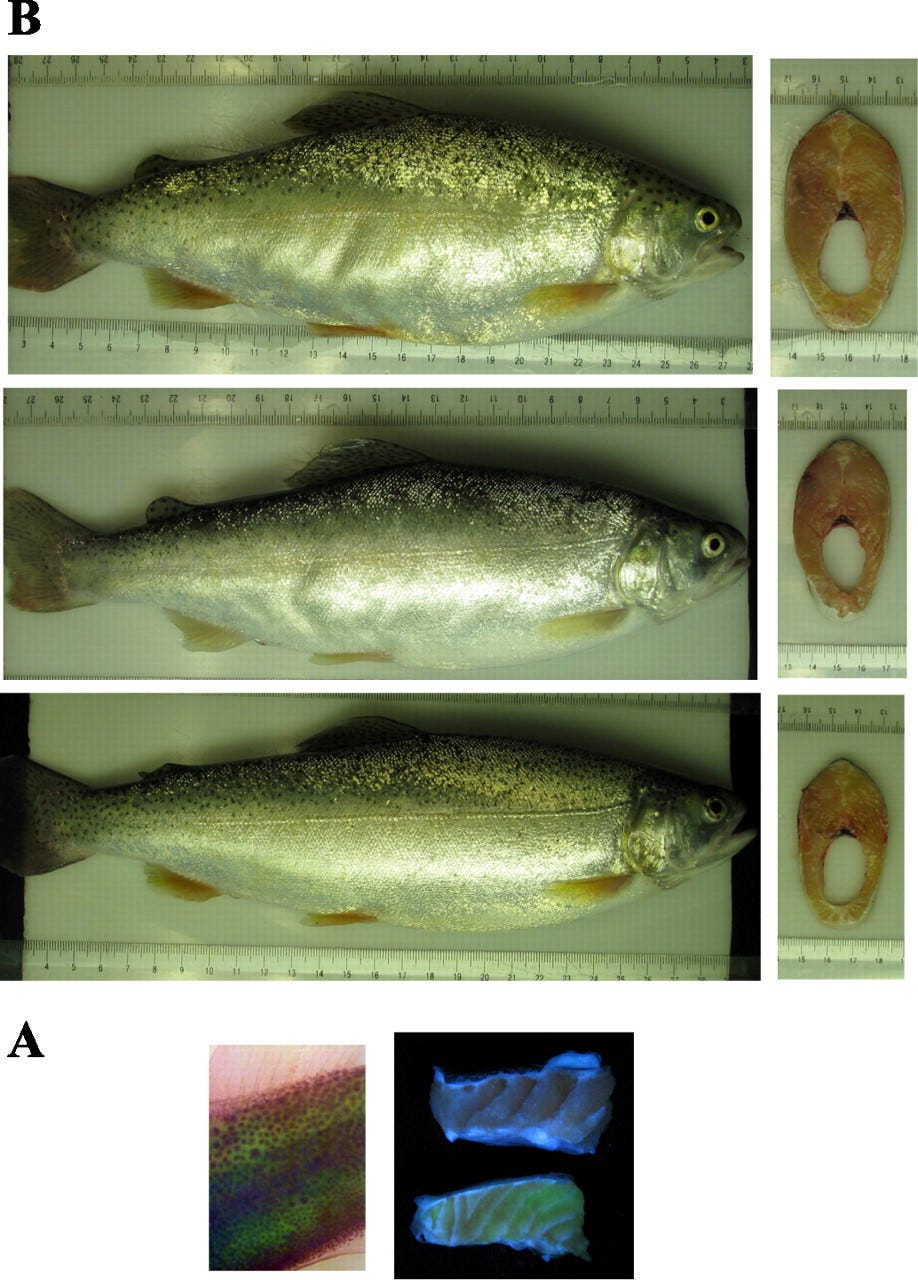
Transgenic rainbow trout10 overexpressing follistatin have increased muscle mass and visibly bulging muscles.
Pigs
Transgenic pigs11 edited to overexpress human follistatin 344 in skeletal muscle were leaner at slaughter (72.95% lean vs. 69.18% wild-type, p = 0.0118) and had thicker muscle fibers than wild type. (Both wild-type and transgenic pigs were slaughtered at 100 kg as per standard Chinese livestock procedure).
Monkeys
An AAV gene therapy containing the follistatin 344 isotype injected into macaque monkeys12 resulted in durable increases in muscle size (25% increase in quadriceps diameter at 20 weeks) along with thicker muscle fibers and greater force generation. Over the next 15 months, the animals showed no abnormal blood work, tissue morphology, or changes in sperm quality or menstruation.
Humans
A 2015 trial13 of an AAV gene therapy of follistatin-344 for Becker Muscular Dystrophy showed improvements in the 6-minute walk test for 4 out of 6 patients and visible areas of muscle hypertrophy close to the injection site.
The more muscle fibrosis each patient had, as observed on an MRI, the less improvement they saw from gene therapy. (This is encouraging for the effectiveness of follistatin in healthy people, who do not generally have macroscopically visible muscle fibrosis before middle age.). Also, the gene therapy reduced fibrosis by half overall, as well as increasing muscle fiber diameter.
Likewise, a 2017 study14 (by the same team at the Nationwide Children’s Hospital) found that an AAV gene therapy of follistatin-344 improved walk test outcomes in 4 out of 6 patients with inclusion body myositis, relative to the controls, who continued to deteriorate over a 2-year followup. As before, the gene therapy reduced fibrosis and increased muscle fiber diameter.
Tiny, Ohio-based Milo Biotechnology developed this gene therapy — they don’t even seem to have a website. They were still in business as of this 2020 article on Ohio’s gene therapy scene, but their founder appears to have left the company in late 2023 and I can’t see any more recent sign of it.
Minicircle DNA Vectors
Minicircle is named for the minicircle, their weird new form of gene therapy.
Their one-pager describes a minicircle as a DNA plasmid, “complexed with a transformation polymer that helps the DNA efficiently get into fat cells”. There is also a “genetic killswitch” encoded into the DNA allowing the plasmids to be destroyed by the common antibiotic tetracycline.
Is this a thing?
Apart from cell therapies that directly administer genetically modified cells, the vast majority of clinical gene therapies, including all four FDA- and EMA-approved ones,15 use adeno-associated viruses (AAVs) to get the DNA into cells.
Minicircle DNA plasmids, by contrast, are not much used in clinical gene therapy, but they are a known technique, first published in 1997.16
You can get gene transfer into cells even with regular DNA plasmids, which are circles of DNA. Electroporation, or exposing cells to electric fields, can make their membranes more permeable so that plasmids get in. Plasmid-based gene therapies have been entered into clinical trials many times; the problem is that they’re less efficient at causing gene expression than viral-vector gene therapies.
But non-viral-vector gene therapies made of “pure” DNA have many advantages, if they can be made to work better:
unlike viral-vector gene therapies, DNA plasmids (and other DNA shapes like minicircles) are never inserted into the genome. Viral vectors sometimes insert off-target effects into the genome, which last forever as cells pass those changes down when they replicate. Virus-free DNA vectors are free of those off-target effects and naturally “dilute” away over time as cells divide, so they’re inherently reversible.
Viral-free DNA gene vectors are cheaper to make, ship, and store than viral and RNA-based vectors.
Viral-free DNA gene vectors are far less likely to provoke a side-effect-heavy immune reaction than viral vectors.
The main problem with DNA plasmids as gene therapies is their poor performance — but there’s a whole zoo of experimental new types of supercoiled DNA gene therapy modalities, including minicircles, which are hypothesized to have better transfection efficiency than traditional plasmids.17
Minicircles, a “supercoiled” form of circular DNA using proteins and sequences derived from E. coli, are extra-small, lacking some of the genetic sequences borrowed from bacteria that are present in plasmids and not necessary for gene transfer. These superfluous sequences are believed to reduce gene transfer into cells — without them, minicircles are more efficient than plasmids.18
There have been several examples in animals showing that minicircle gene therapy performs better than plasmid DNA gene therapy, for instance a 2012 mouse study19 comparing injecting plasmid GFP (green fluorescent protein) intramuscularly to minicircle GFP: fluorescence was 2-10x higher in the minicircle mice.
Other studies find even bigger effects, especially in the long term — mice injected with minicircles had thousands of times higher serum levels of the transgene 120 days after injection, compared to mice injected with a plasmid transgene.20
Minicircles have never been used in a clinical trial so far, but there are lots and lots of preclinical (typically mouse) studies applying them to a variety of genetic diseases, as vaccines for infectious diseases, and as genetic modifications to T-cells for cancer immunotherapies.
They are tricky to purify and manufacture at scale, however21, which may be one reason they haven’t hit the clinic yet.
What about this “transformation polymer” Minicircle mentions that supposedly helps the DNA get into the cells?
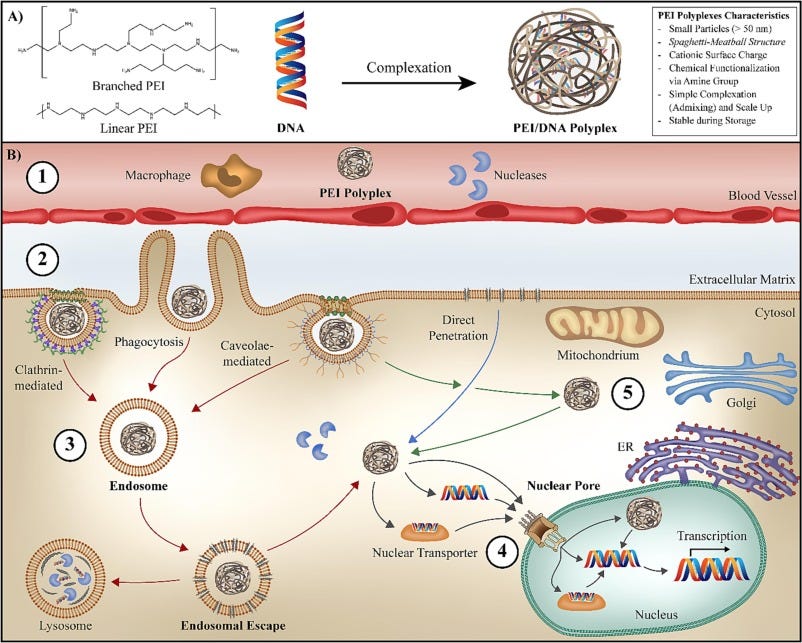
Polymers to aid in gene delivery are a known thing — for instance, PEI (polyethylenimine) has been used since 1995 as the “gold standard”, complexing with DNA in a “spaghetti and meatball” bundle that gets incorporated into cells via endocytosis.
PEI has been used in lots of clinical trials, mostly cancer vaccines.22
Obviously I don’t know what polymer Minicircle uses, or how well it works, but complexing DNA with polymers isn’t unreasonable a priori.
Bottom Lines
Should follistatin gene therapy increase muscle mass and strength?
Yes — as much as animal trials can ever give us optimism about effects on humans, all signs in animals point to “follistatin works.”
Should minicircle gene therapy be effective?
That’s more of a maybe.
Minicircles are real science, and there are real reasons to think they should have advantages over viral vectors on the one hand, and DNA plasmids on the other.
But they haven’t been used on humans yet.
What’s more of a concern, in my opinion, from the perspective of a person considering signing up for a Minicircle gene therapy, is manufacturing and purification.
Sure, in principle, this is an approach that makes sense, but do you trust these guys to have pure reagents, to have designed their minicircles and the associated polymers etc to be safe, and so on?
Without being able to lean on the usual regulatory requirements, you need to do more diligence about whether the stuff that you’re getting injected in your body is what they say it is, whether they have the appropriate equipment & tests at their facilities to make sure it’s not contaminated, etc.
The founder, Mac Davis, does claim to have experience with GMP manufacturing of gene therapies, which is reassuring…but they don’t seem to have any “graybeards” from the pharma industry or even PhDs on the team, which is a negative sign.
If you’re seriously interested, it’s probably worth contacting the team and asking technical questions.
This is something where the absolute worst-case scenario is nontrivial — "what’s the worst thing that could happen if I were injected with some unknown fluid?” — so your risk assessment should depend on things like whether you trust the team to be honest, where they’re getting their minicircles manufactured, what’s in that mysterious “polymer”, whether they use good sanitation practices for storage, etc.
But in principle, are they doing things that make sense and could work?
Absolutely.
This is a straightforwardly plausible and promising approach.
There’s an obvious reason that follistatin gene therapy hasn’t yet been done in traditional clinical trials — the FDA is not interested in helping healthy people get swole — and as far as I can see, the evidence that it would work is as good as you can ever get short of having those clinical trials already.
Using minicircles rather than a more established viral gene delivery mechanism does add some “technical risk”, but it also could reduce costs, side effects, and simplify manufacturing and storage, so it doesn’t seem crazy.
As always, I am a layman, so there could be problems I haven’t noticed — if you know of any, please let me know in the comments!
Rodino‐Klapac, Louise R., et al. "Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease." Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 39.3 (2009): 283-296.
Winbanks, Catherine E., et al. "Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin." Journal of Cell Biology 197.7 (2012): 997-1008.
Han, Xiuqing, et al. "Mechanisms involved in follistatin‐induced hypertrophy and increased insulin action in skeletal muscle." Journal of Cachexia, Sarcopenia and Muscle 10.6 (2019): 1241-1257.
Barbé, Caroline, et al. "Role of IGF-I in follistatin-induced skeletal muscle hypertrophy." American Journal of Physiology-Endocrinology and Metabolism 309.6 (2015): E557-E567.
Castonguay, Roselyne, et al. "Follistatin-288-Fc fusion protein promotes localized growth of skeletal muscle." Journal of Pharmacology and Experimental Therapeutics 368.3 (2019): 435-445.
Zheng, Hui, et al. "Follistatin N terminus differentially regulates muscle size and fat in vivo." Experimental & molecular medicine 49.9 (2017): e377-e377.
Gangopadhyay, Samudra S. "Systemic administration of follistatin288 increases muscle mass and reduces fat accumulation in mice." Scientific reports 3.1 (2013): 2441.
Jaijyan, Dabbu Kumar, et al. "New intranasal and injectable gene therapy for healthy life extension." Proceedings of the National Academy of Sciences 119.20 (2022): e2121499119.
Gilson, Hélène, et al. "Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin." American Journal of Physiology-Endocrinology and Metabolism 297.1 (2009): E157-E164.
Medeiros, Erika F., et al. "Overexpression of follistatin in trout stimulates increased muscling." American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 297.1 (2009): R235-R242.
Chang, Fei, et al. "The transgenic expression of human follistatin-344 increases skeletal muscle mass in pigs." Transgenic research 26 (2017): 25-36.
Kota, Janaiah, et al. "Follistatin gene delivery enhances muscle growth and strength in nonhuman primates." Science translational medicine 1.6 (2009): 6ra15-6ra15.
Mendell, Jerry R., et al. "A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy." Molecular Therapy 23.1 (2015): 192-201.
Mendell, Jerry R., et al. "Follistatin gene therapy for sporadic inclusion body myositis improves functional outcomes." Molecular Therapy 25.4 (2017): 870-879.
Kohn, Donald B., Yvonne Y. Chen, and Melissa J. Spencer. "Successes and challenges in clinical gene therapy." Gene Therapy 30.10 (2023): 738-746.
Darquet, A. M., et al. "A new DNA vehicle for nonviral gene delivery: supercoiled minicircle." Gene therapy 4.12 (1997): 1341-1349.
Hardee, Cinnamon L., et al. "Advances in non-viral DNA vectors for gene therapy." Genes 8.2 (2017): 65.
Dietz, Wynette M., et al. "Minicircle DNA is superior to plasmid DNA in eliciting antigen-specific CD8+ T-cell responses." Molecular therapy 21.8 (2013): 1526-1535.
Chabot, S., et al. "Minicircle DNA electrotransfer for efficient tissue-targeted gene delivery." Gene therapy 20.1 (2013): 62-68.
Chen, Zhi-Ying, et al. "Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo." Molecular therapy 8.3 (2003): 495-500.
Alves, Cláudia PA, Duarte Miguel F. Prazeres, and Gabriel A. Monteiro. "Minicircle biopharmaceuticals–an overview of purification strategies." Frontiers in Chemical Engineering 2 (2021): 612594.
Casper, Jens, et al. "Polyethylenimine (PEI) in gene therapy: Current status and clinical applications." Journal of Controlled Release 362 (2023): 667-691.







How many of their potential customers do you think are capable of asking enough "technical questions" to satisfy their concerns? You're missing several important ones yourself, and you're probably better qualified than ~100% of the people who've already received Minicircle's "treatment".
Here's a couple freebies: minicircles might be 10x more effective than regular plasmids, but how effective were those regular plasmids? No one is questioning the *potential* of follistatin, but try to find a study were the animals weren't genetically engineered to have the gene in every cell in their body, or injected with eye-watering levels of AAV (a somewhat more effective vector than plasmids).
Well, as the founder of a science-based therapy clinic (granted, it is an across-the-border dodgy one) I would not list on my LinkedIn profile a 200 hr Kundalini Yoga certification, like the founder of MiniCircle has. This fact by default should give anyone in their right mind pause as to the type of rigorous scientific practice they run there!